
Approach

Approach
At Bolt, we are leveraging the immune system for a better way to treat cancer
The “Adaptive” immune system, including T cells and B cells, is the normal guardian against cancer. When cancer progresses it is a sign that the adaptive immune system is being overwhelmed. Cancer develops in ways that evade the immune system and suppress an immune response.
To get a better result, we need a new approach. By recruiting the innate immune system we are bringing in reinforcements and trying to initiate an entirely new immune response. The hope is that this help from the innate immune system will be what the adaptive immune system needs to recognize and overcome the cancer.

Macrophage Repolarization
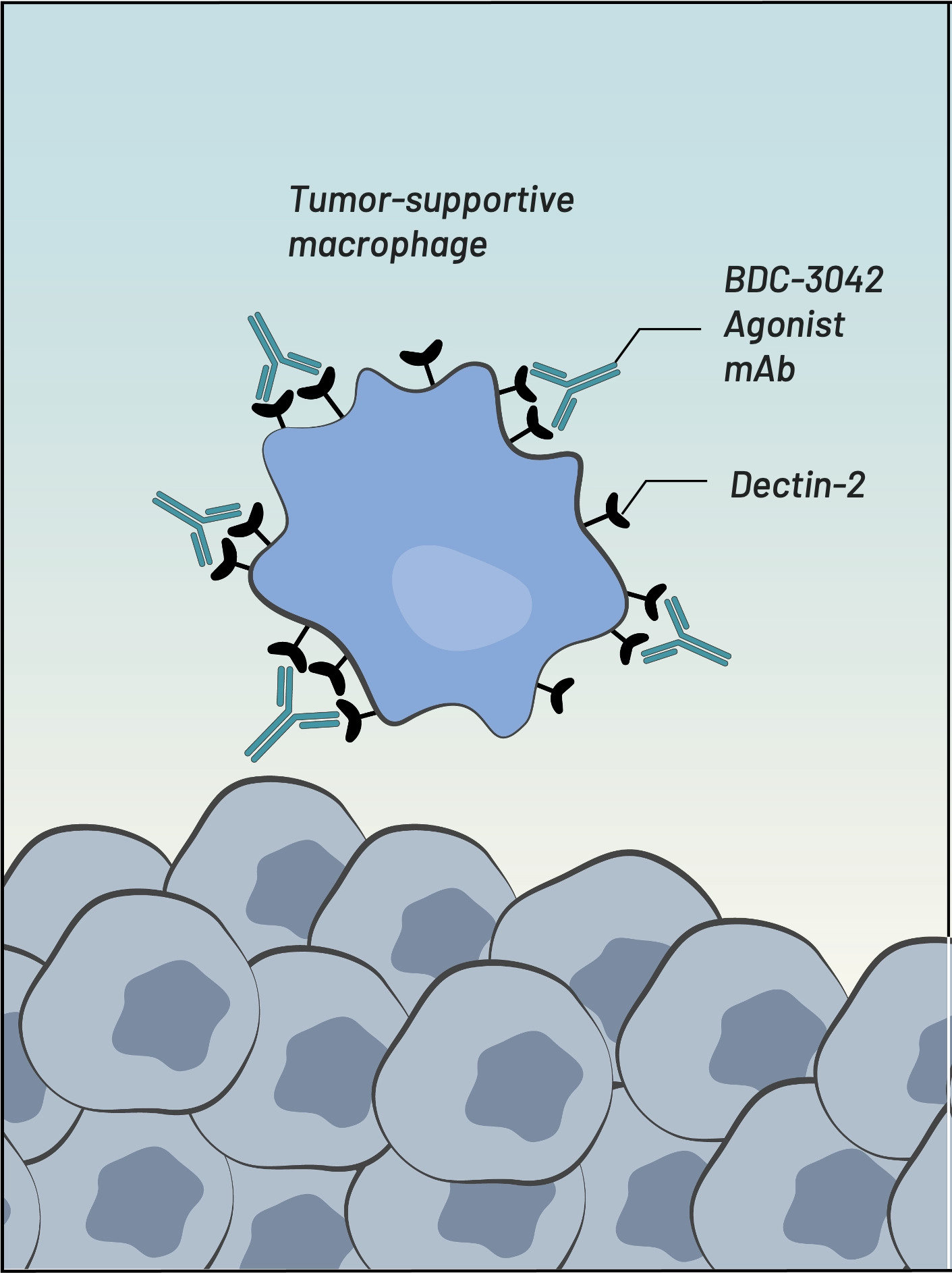
Our BDC-3042 product candidate reawakens myeloid cells to attack tumor cells. We discovered an agonist monoclonal antibody that is capable of binding to and activating a pattern-recognition receptor (known as Dectin-2) on tumor-associated macrophages (TAMs) in a broad range of solid tumors. BDC-3042 works by repolarizing TAMs from tumor-supportive to tumor-destructive. It is currently being evaluated in a Phase 1 trial.
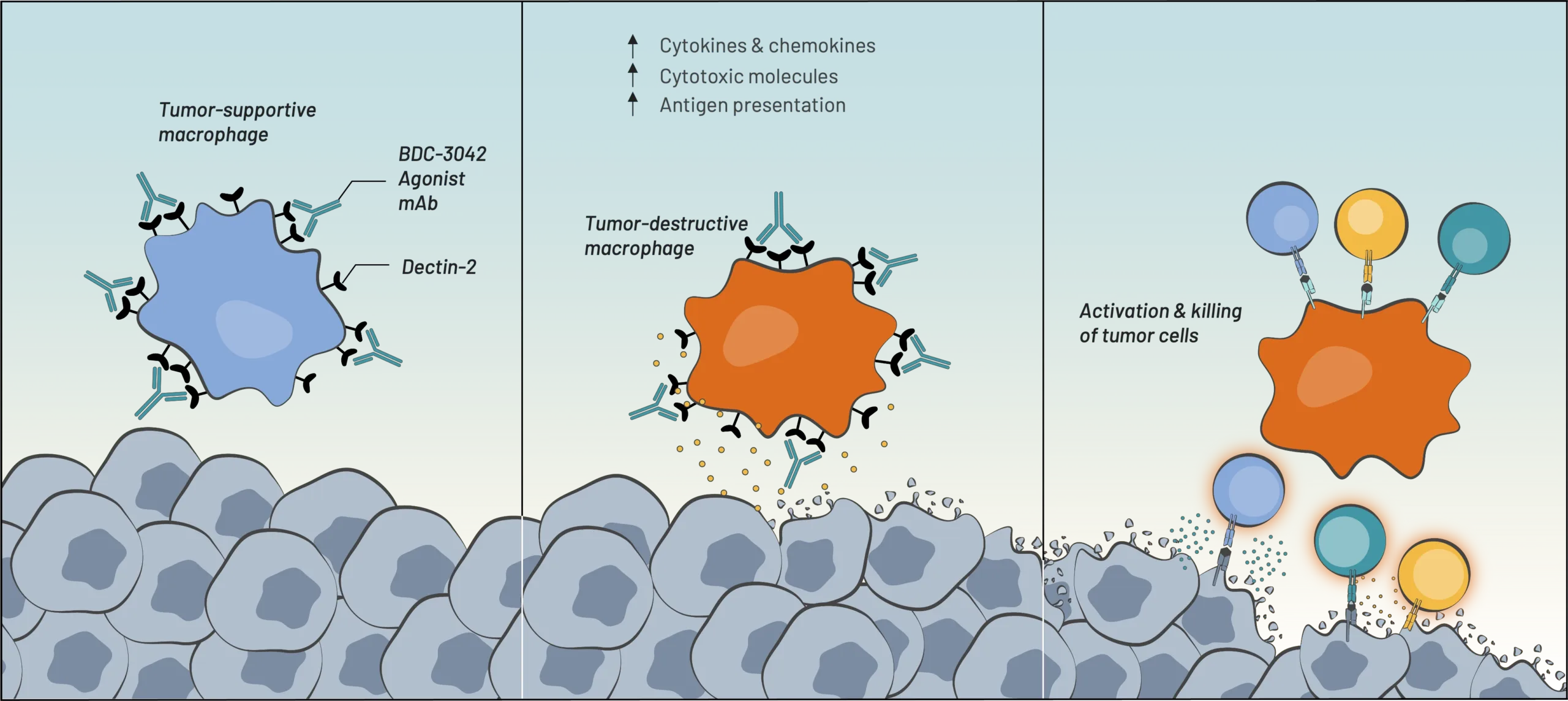
BDC-3042 binds to Dectin-2 & activates macrophages
Macrophages transform
into tumor-destructive macrophages
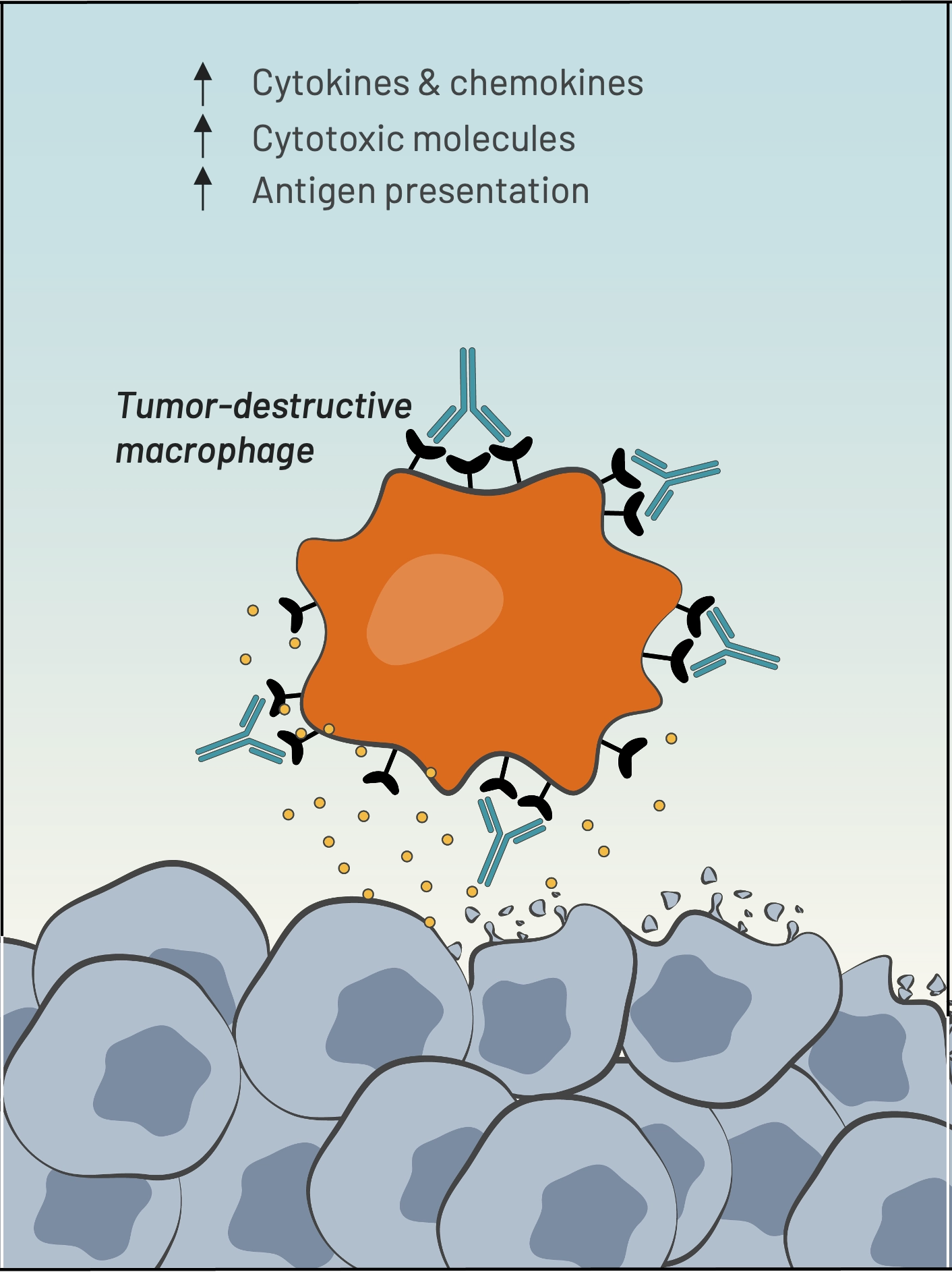
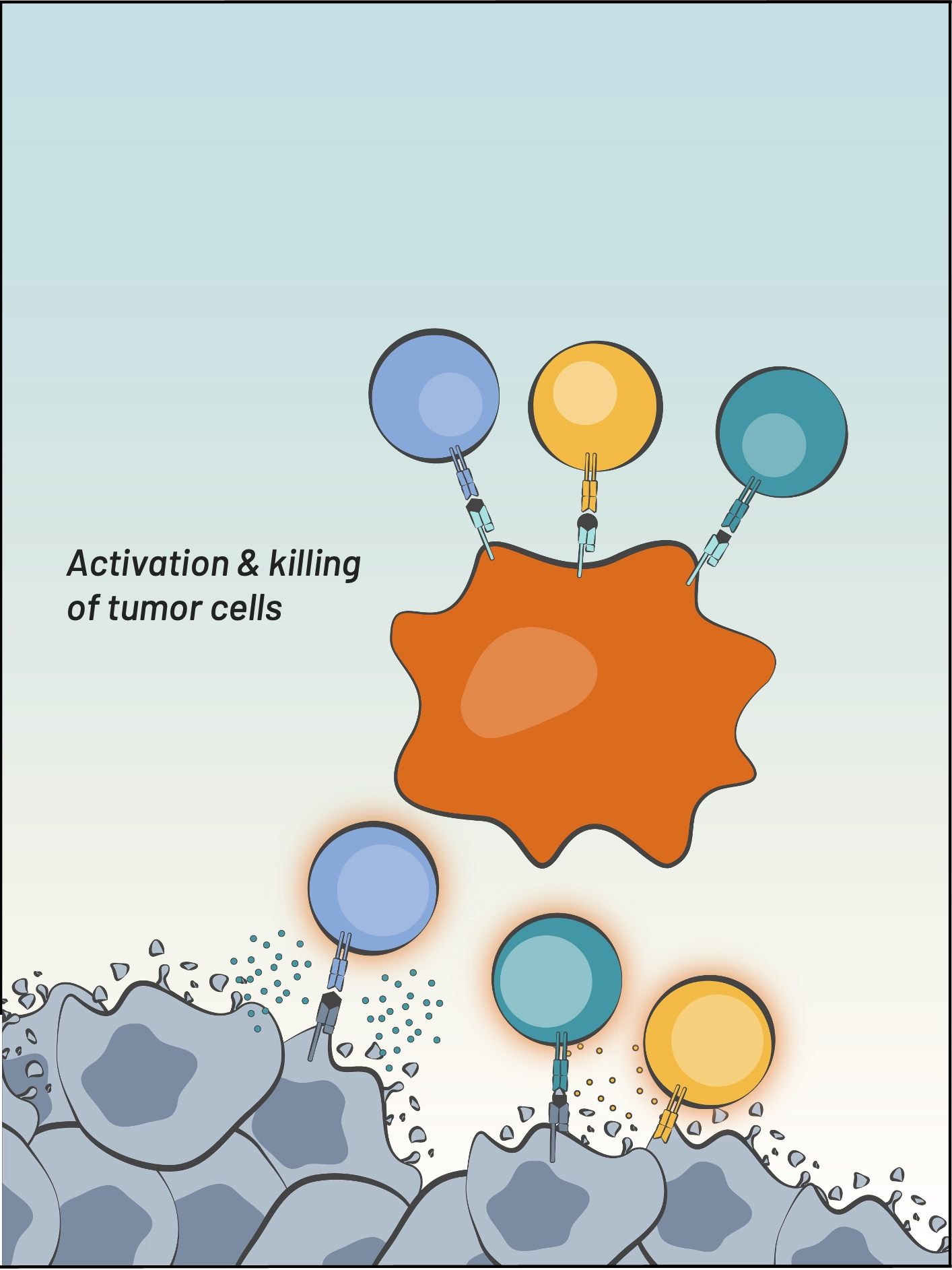
CD8+ T cell &
NK cell recruitment,
activation, & tumor killing
[Innate Immunity]
![]()
[Adaptive Immunity]
BDC-3042 binds to Dectin-2 & activates macrophages
Macrophages transform into tumor-destructive macrophages
[Innate Immunity]
CD8+ T cell &
NK cell recruitment,
activation, & tumor killing
[Adaptive Immunity]

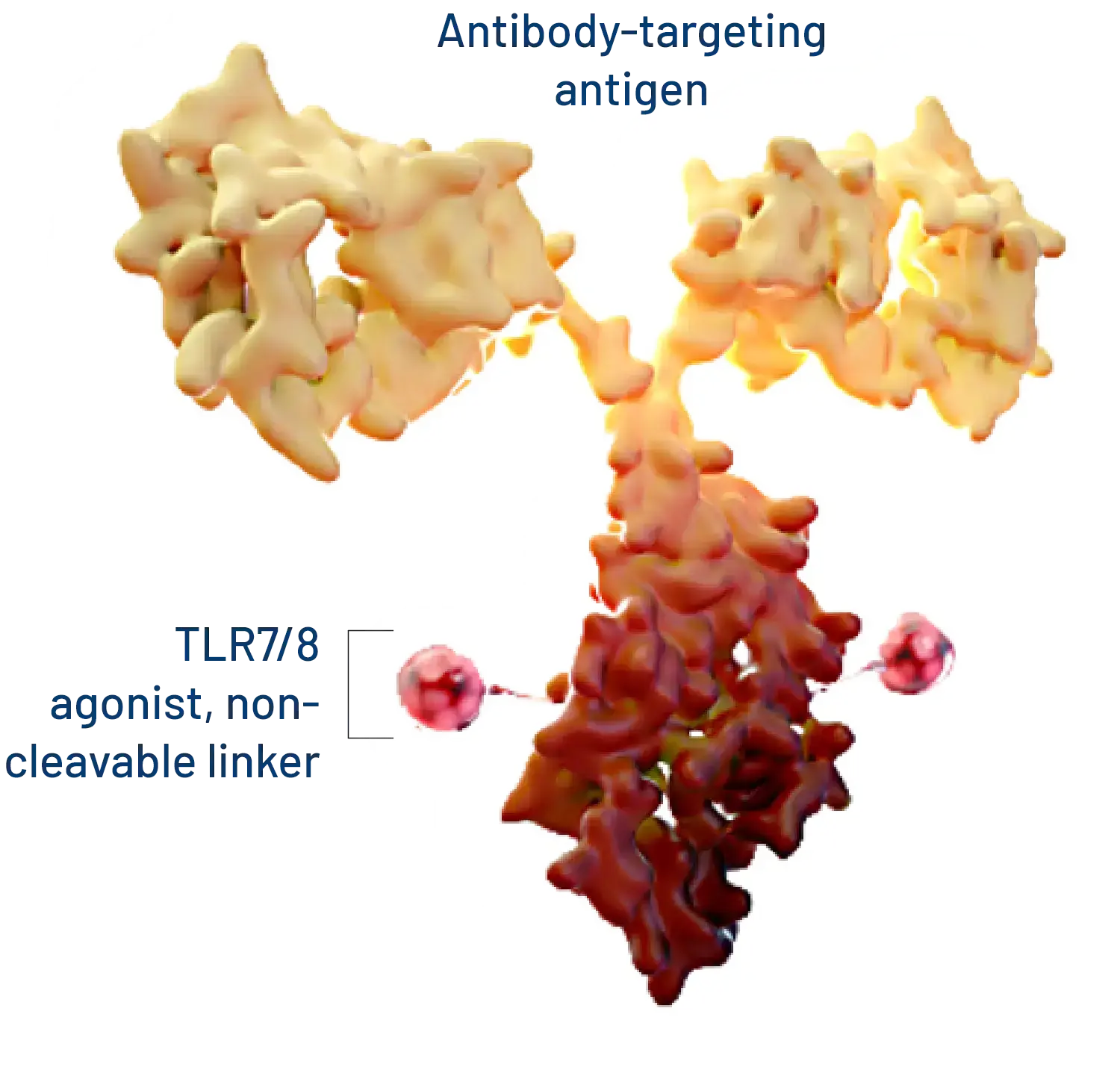
Boltbody™ ISAC Platform
Despite the power of newer cancer treatments, their utility is often reduced due to side effects from a drug’s impact on healthy cells and from treatment-generated systemic immune responses that aren’t tumor-specific enough. Threading this needle to treat cancer with minimal side effects is made more difficult because cancer originates from a person’s own cells and every cancer is unique. Our approach works with a person’s body by teaching the immune system to recognize and kill cancer in a way that is immediately personalized to each patient’s tumor.
Our Immune-Stimulating Antibody Conjugate (ISAC) platform technology enables tumor-specific activation of the innate immune system which generates a durable anti-cancer adaptive immune response. ISACs use the precision of antibody targeting to harness the power of the innate immune system, which reprograms the tumor microenvironment and invokes a new anti-tumor immune response that can spread to the adaptive immune system.
KEY FEATURES
Only ISAC platform with emerging clinical validation
Single-agent activity demonstrated preclinically with many different tumor targets
Ability to address difficult-to-treat solid tumors, including those refractory to current treatments
Pipeline engine that is widely applicable

KEY FEATURES
Only ISAC platform with emerging clinical validation
Single-agent activity demonstrated preclinically with many different tumor targets
Ability to address difficult-to-treat solid tumors, including those refractory to current treatments
Pipeline engine that is widely applicable
Our technology teaches the immune system to recognize and kill cancer in a way that is immediately personalized to each patient’s tumor
Our Boltbody ISACs are delivered systemically but act locally in the tumor microenvironment by triggering a localized anti-tumor immune cascade. This process enables the patients’ own immune system to determine which are the relevant T cells to mobilize for tumor destruction and subsequent immunosurveillance, so an off-the shelf Boltbody immunotherapeutic can deliver a personalized therapeutic outcome. We believe that the development of systemic immunological memory will generate durable therapeutic responses for patients with cancer. Our next-gen Boltbody ISAC BDC-4182 is under development for gastric, gastroesophageal, and pancreatic cancers. We anticipate initiating a clinical trial in 2025.
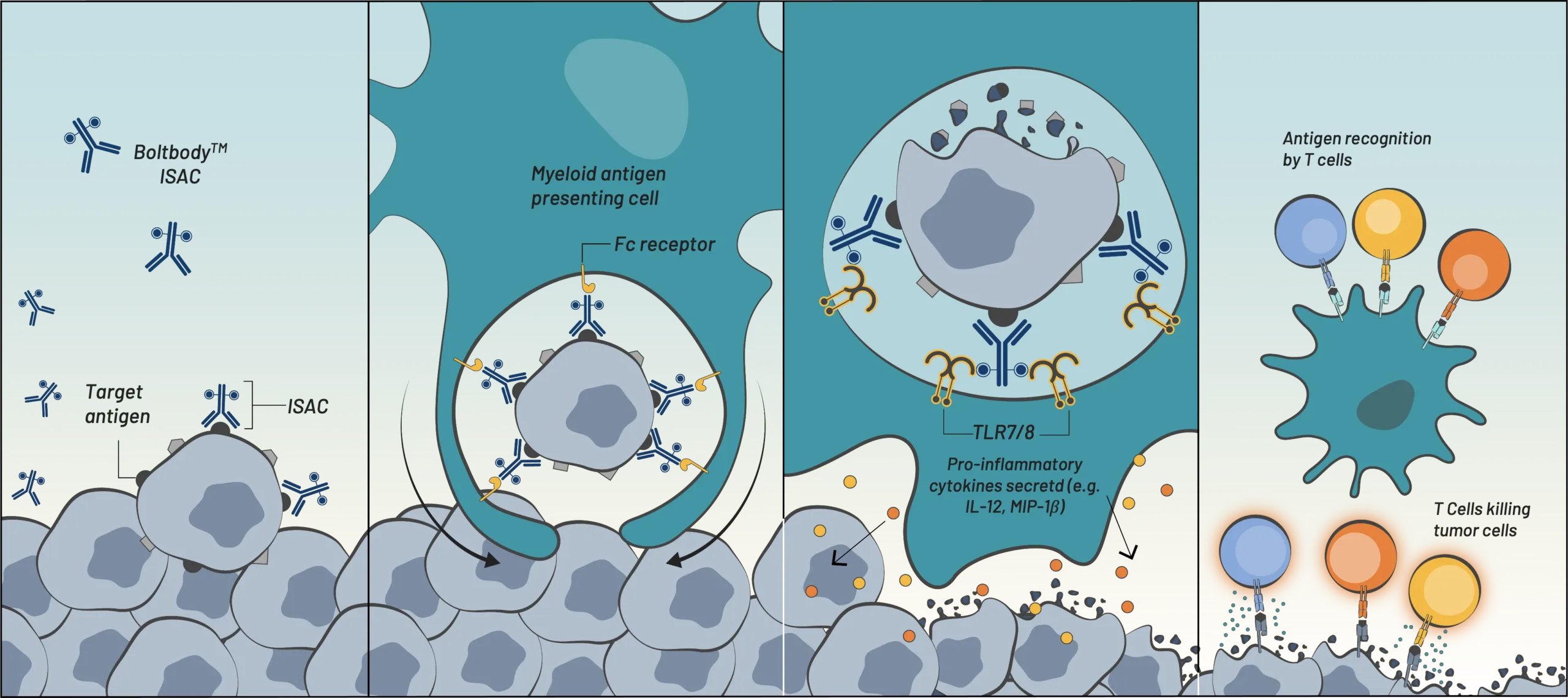
Boltbody ISAC binds
tumor antigen
Fc receptor-mediated
phagocytosis by myeloid cells
TLR7/8-mediated activation
& tumor antigen processing
Antigen presentation,
T cell recruitment,
& tumor cell killing
[Innate Immunity] ![]() [Adaptive Immunity]
[Adaptive Immunity]
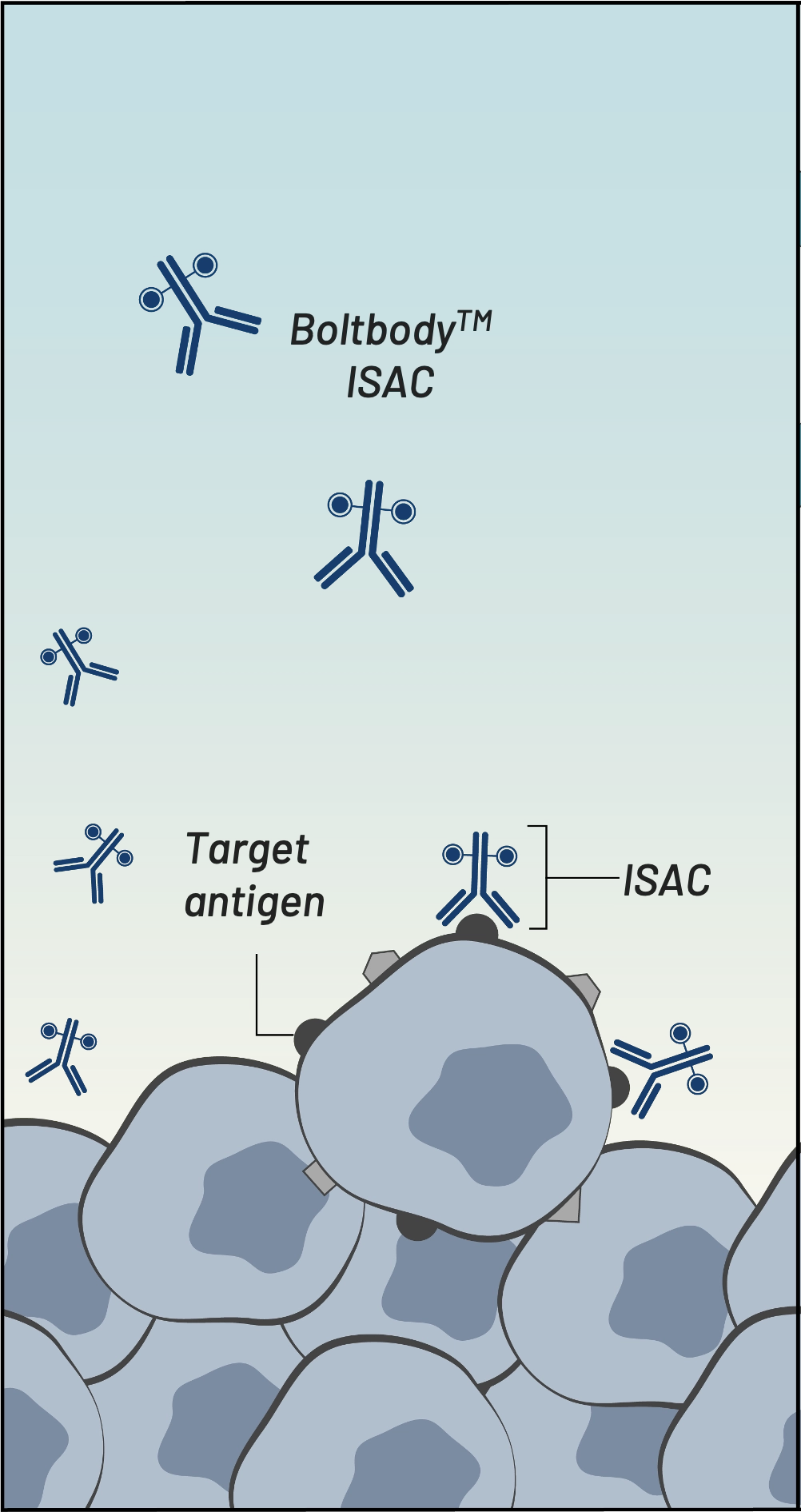
Boltbody ISAC binds tumor antigen
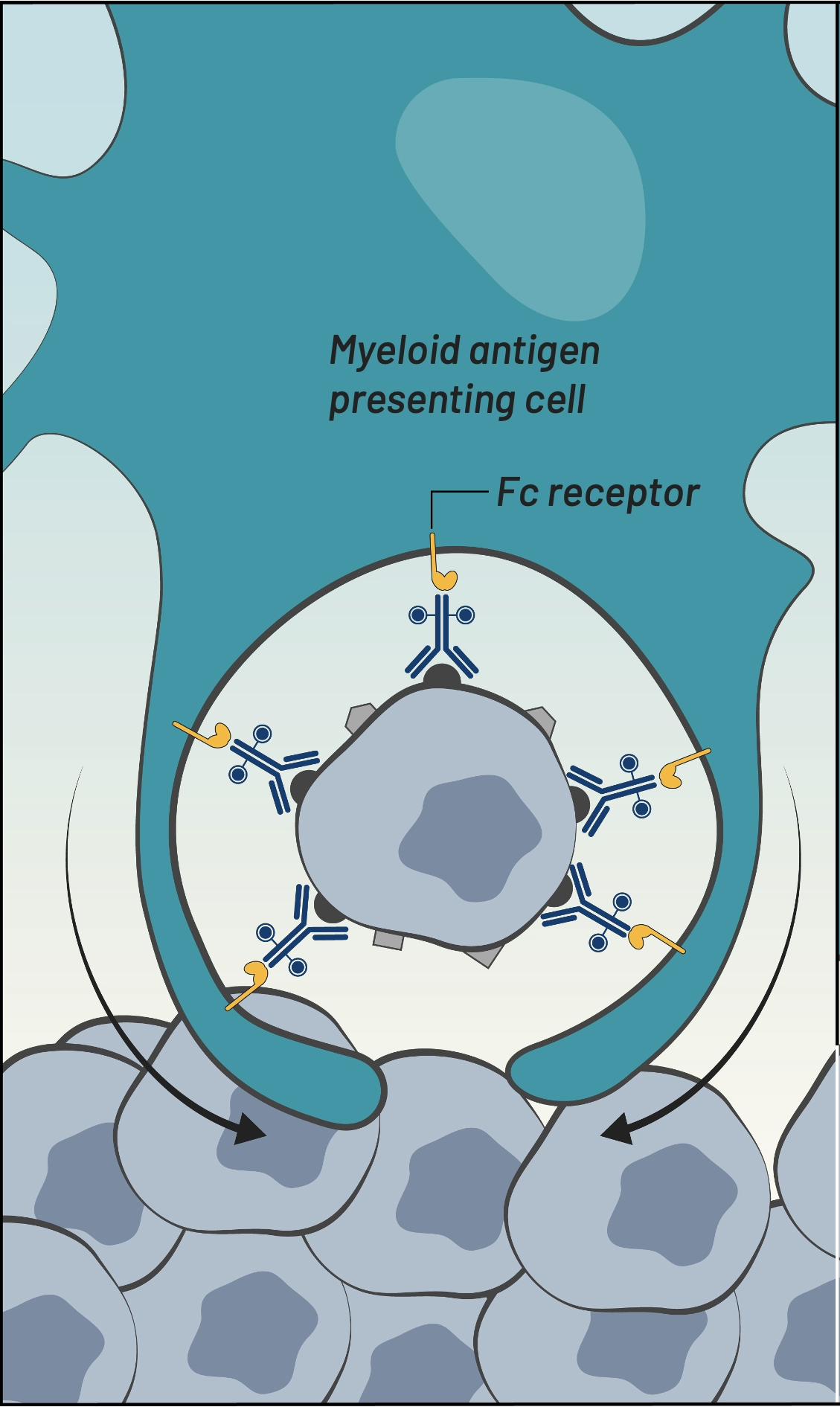
Fc receptor-mediated phagocytosis by myeloid cells
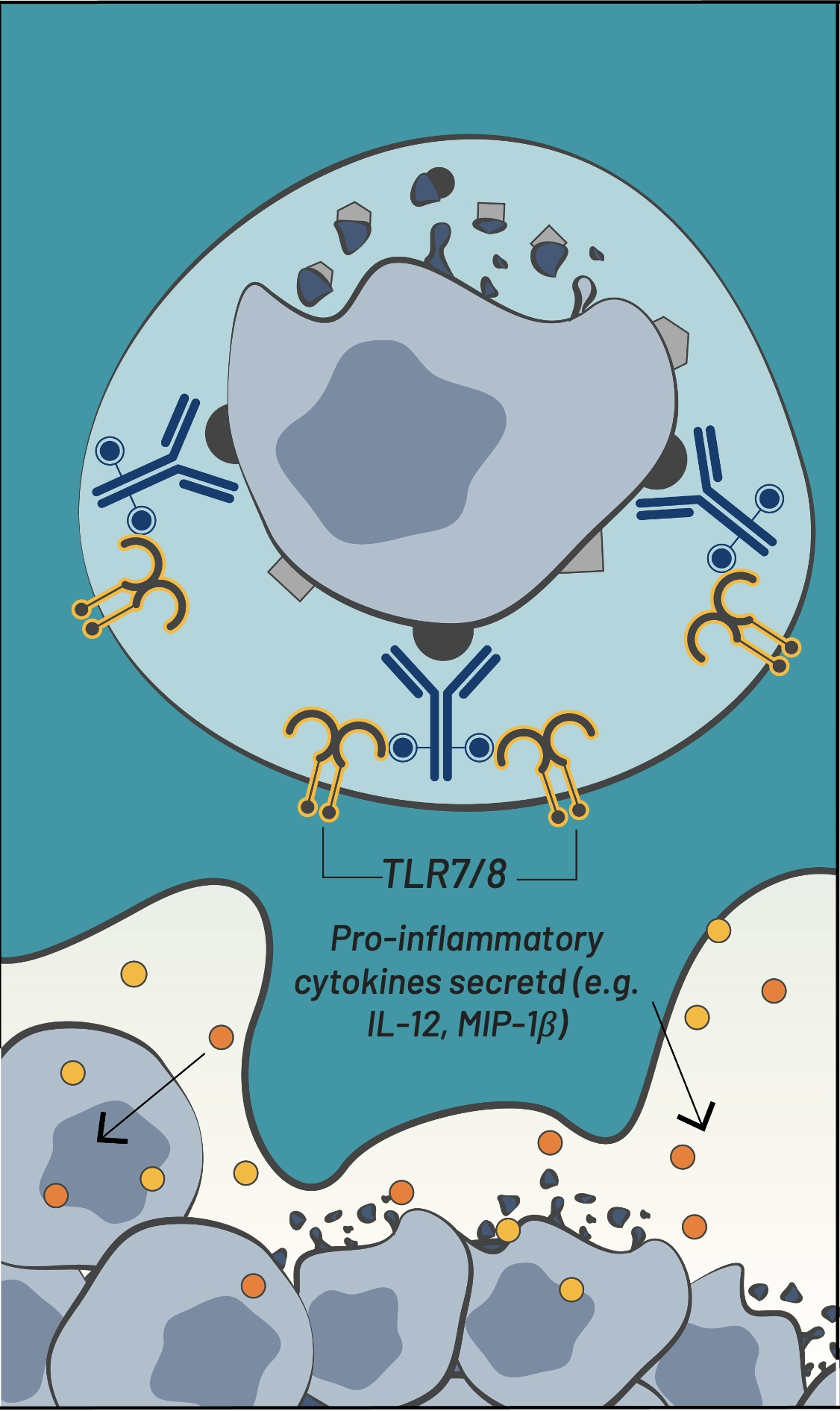
TLR7/8-mediated activation & tumor antigen processing
[Innate Immunity]

Antigen presentation, T cell recruitment, & tumor cell killing
[Adaptive Immunity]
